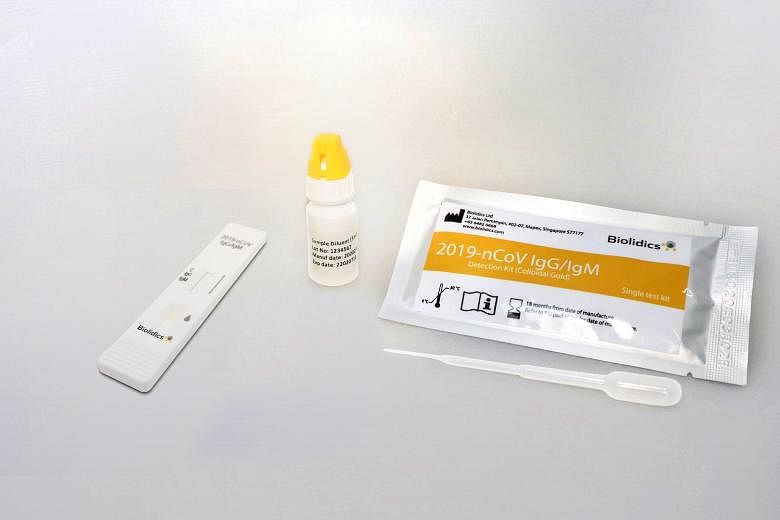
SINGAPORE (THE BUSINESS TIMES) - Singapore-based cancer diagnostics company Biolidics can now distribute, market and sell its Covid-19 rapid test kits in the US, it said in a bourse filing on Monday (April 20)
The Catalist-listed firm completed its listing with the US Food and Drug Administration (FDA) on April 17.
Its test kits are allowed for use by clinical laboratories or healthcare workers for point-of-care testing, but not for home testing.
In its filing, Biolidics said that its test kits are solely used for identifying antibodies to SARS-CoV-2, which is the virus that causes Covid-19.
Its test reports must also state that the tests have not been reviewed by the FDA. A negative result from testing does not rule out a SARS-CoV-2 infection, while a positive result could be due to past or present infections with non-SARS-CoV-2 strains, such as coronavirus HKU1, NL63, OC43, or 229E.
The company's test kits are already allowed for sale in the European Union. It has also received approval for the test kits from Singapore's Health Sciences Authority and the Food and Drug Administration of the Philippines.
Biolidics shares were flat at 31.5 cents at Friday's close.